Database
We store the genetic and clinical data of thousands of patients in the Netherlands with metastatic cancer in the Hartwig Medical Database. The genetic data are generated using OncoAct. The hospitals that treat the patients provide the clinical data, including the treatment outcomes. The data is stored, processed and provided to third parties. It is the largest database of its kind in the world.
Research for improving cancer treatment
The data is anonymized and made available free of charge to cancer research institutes and hospitals. It is used by research groups all over the world.


Purpose of the database: to discover new biomarkers
The data is helping researchers to gain a better understanding of cancer. The huge amount of patient data enables them to recognize patterns. By establishing a relationship between DNA mutations, tumor characteristics, drugs and efficacy, researchers can discover new biomarkers. The outcome of this research are new treatment options, and improved treatment efficacy.
Generating data with WGS
We analyze each patient’s DNA in both tumor and We generate the genetic data by Whole Genome Sequencing (WGS). By comparing, the generated genetic data of patient’s DNA in tumor and blood sample, mutations and other tumor characteristics are found.

Analyzing the data
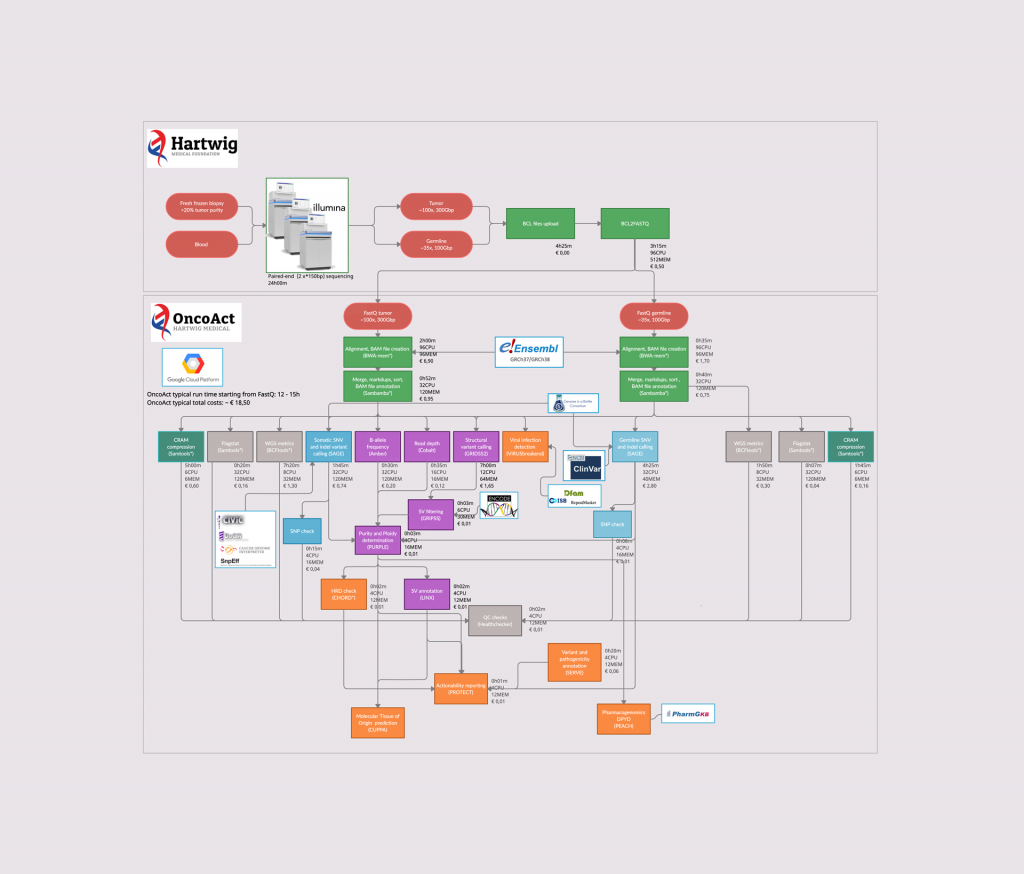
We developed a state of the art IT pipeline for the bioinformatic data analysis. We have built various software tools, most of which were developed in house. This allows us to analyze the WGS data. All software, including all the relevant documentation relating to the IT pipeline, is accessible on GitHub.
The Platinum system and tools used in the Hartwig Medical Foundation IT pipeline (click on the image to enlarge it)
Content of the database
The WGS data of over 5,000 patients are stored in the Hartwig Medical Database. This is the largest database of metastatic tumor data obtained with WGS in the world. The uniqueness of the database consists of combining genetic data with treatment and treatment outcome data.
The incidence of solid tumors in the Netherlands is reflected in the quantity and frequency of the tumor types in the database.
In addition to molecular data, the database also contains an ever-growing list of tumor characteristics. Our processes are standardized from sample preparation to patient reporting assuring a highly consistent dataset.
Researchers can view an anonymized and up-to-date subset of data from the Hartwig Medical Database in the Hartwig Data Catalogue.
Data stored and published with due care
1. Data stored and published with due care
Of course, we have a strict privacy policy that governs the use of patients’ personal data.
2. Basic principles
We have established basic principles relating to the social, ethical, legal and contractual consequences of publishing this data. Data Access Requests by researchers are reviewed by our Scientific Board and Data Access Board. For We have established basic principles on social, ethical, legal and contractual aspects of using patients’ data. Data Access Requests by researchers are reviewed by our Scientific Board and Data Access Board. The research must contribute to improve cancer treatment and must serve the public interest. We provide a specific subset of data geared to the specific needs of the research project (purpose principle).
3. Standard application procedure
Those who apply for access to data must go through a standard procedure that includes signing a license agreement and pledging to abide by the rules for publication.
4. European privacy legislation
In accordance with European privacy legislation specific requirements apply to the provision of data to parties located in a country outside the European Economic Area (EEA).
5. Rules for publication
Researchers who use data provided by Hartwig Medical Foundation must explicitly state this use in all publications.

The fact that the CPCT, together with Hartwig Medical Foundation, has managed to bundle data, knowledge and expertise from almost all Dutch hospitals is unique and now also deserves international attention.